Radiation can be natural, like the radiation that comes from the sun, and it can be artificial or induced, like the radiation that we use in diagnosing and treating cancer.
It can also be classified as non-ionising, like radio waves and microwaves, or as ionising, radiation that has so much energy it can affect the atoms in living things. This can come from X-ray machines and cosmic particles from space.
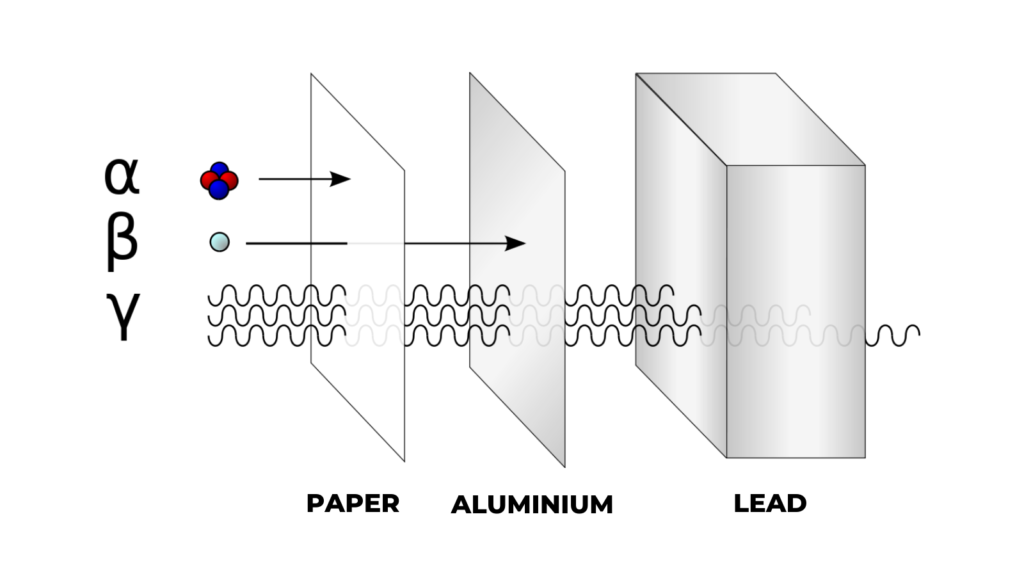
Under ionising radiation comes three types of emitted particles: alpha, beta and gamma. Each of them has a different ability to penetrate matter and while alpha particles carry a positive charge, beta has a negative charge and gamma rays are neutral.
Alpha (α)
Alpha particles come from the decay of the heaviest radioactive elements like uranium and radium. But even with the high energy, each particle is so heavy that the energy dwindles over short distances and cannot travel very far from the atom.
Alpha particles’ massive size means they have very low penetration power. A piece of paper or the outside layer of the human skin can completely stop them. However, if a person inhales or absorbs alpha emitters from a wound, it can damage sensitive living tissue.
Now, where do alpha particles come from?
Many of them come naturally from the environment–from rocks, soil and water. These are also artificially produced in a nuclear reactor.

Beta (β)
While alpha particles are positively charged and heavy, beta particles are negatively charged, smaller and fast-moving. In some cases, its intensity can cause burns and if inhaled or ingested can cause more damage to cells and organs.
Beta particles are more penetrating than alpha particles and can pass through thicker materials like paper. What can stop beta particles is thin aluminum shielding or a layer of clothing.
Like alpha particles, these are emitted naturally or artificially, through the usually man-made nuclear fission. Strontium-90 or strontium-89 is used in the treatment of eye and bone cancers while fluorine-18 is used in PET scans.

Gamma (γ)
Lastly, gamma rays are a form of electromagnetic radiation similar to X-rays. Unlike alpha and beta, gamma particles have no mass and charge and can penetrate most common objects including metals. What can only stop gamma rays are thick lead and concrete.
Often emitted along with alpha or beta, gamma rays can be a hazard with their ability to completely pass through the human body and damage tissue and DNA.
But where do we get doses of gamma radiation?

X-rays
Similar to gamma rays, X-rays can pass through the human body and are used in medical imaging. However, different parts absorb X-rays in different amounts. For example, bones absorb more rays than the tissues around them that’s why bones have a clearer image on an X-ray film.

Radiation Monitoring Services
Radiation is all around us and is useful in different industries like medicine, aviation and nuclear. For any organisation working with radiation, safety officers must ensure that the dose received by the staff and nearby communities is as low as reasonably achievable.
SensaWeb helps improve the safety and security of these organisations by providing real-time radiation data. Having real-time data provides regulators with confidence that a facility is meeting its licence requirements under the region’s Radiation Safety Standards.
Looking for area radiation monitors or personal radiation monitoring devices? SensaWeb got you covered. With our monitors, you can easily detect and interdict radioactive materials. Connect with us here or through our email address: info@sensaweb.com.au. You can also call us at +61 415 409 467.